Grade 12 Chemistry(窗了)
本文最后更新于:December 28, 2025 11:38 PM
懒得写了,遛了遛了
A note for my grade 12 chem class.
因为这学科太tm恶心了,我怕我不认真搞会凉所以专门弄一篇呜呜呜
Grade 9-11 Review
Significant digit
Adding and Subtracting
小数点最少的那个
Multiplying and Dividing
significant digit最少的那个
Nomenclature
| oxyacid | anion | anion name | oxyacid |
|---|---|---|---|
| HClO | ClO- | hypochlorite | hypochlorous acid |
| HClO2 | ClO2- | chlorite | chlorous acid |
| HClO3 | ClO3- | chlorate | chloric acid |
| HClO4 | ClO4- | perchlorate | perchloric acid |
1) -ic acid
HNO3 nitric acid
H2CO3 carbonic acid
HClO3 chloric acid
H3SO4 sulphuric acid
H3PO4 phosphuric acid
2)per-ic acid (+1 oxygen from -ic)
HClO4 percholric acid
3) -ate ion (-1/2/3 H from -ic)(not stable)
NO3- nitrate ion
HCO3- hydrogen carbonate / bicarbonate
- CO32- carbonate
HClO2- chlorate
HSO4- hydrogen sulphate / bisulphate
- SO42- sulphate
H2PO4- dihydrogen phosphate
- HPO42- hydrogen phosphate / biphosphate
- PO43- phosphate
CH3COO acetate
4) per-ate ions (-1 H from per-ic)
ClO4- perchlorate ion
5) -ous acid (-1 O from -ic)
HNO2 nitrous acid
HClO2 clorous acid
H2SO3 sulphurous acid
H3PO3 Phosphurous acid
6) -ite ion (-1/2/3 H remove from -ous)
NO2- nitrite ion
ClO2- cholorite
HSO3- hydrogen sulphite / bisulphite
- SO32- sulphite
H2PO3- dihydrogen phosphite
- HPO32- hydrogen phosphite / biphosphite
- PO33- phosphite
7) hypo-ous (-1 O from -ous)
HClO hypochlorous acid (Halogens)
8) hypo-ite (-1 H from hypo-ous)
ClO- hypochlorite acid
Relating Mass to Moles
m = mass(g)
n = number of moles
Mm =molar mass(g)(Some people use MM as abbreviation
$n=\frac{m}{Mm}$ or $m=n\times Mn$
N = number of individual particle
n = number of moles
NAV = Avogadro’s Number
$N=n\times N_{AV}$
for solutions:
$n = c\times v$
for gases:
P = pressure(kPa)
V = volumn(L)
n = Number of moles of gas(mol)
T = Temperature
R = Gas constant = $\frac{8.31kPa\times L}{mol\times K}$
$PV=nRT$
$n=\frac{PV}{RT}$
Types of Reactions
Synthesis Reaction
X + Y → XY
1) Simple oxidation reactions (heating with oxygen)
Metal or non-metal + oxygen → an oxide

2) Binary compound formation
Metal or non-metal + oxygen → binary compound

3) Acid and Base formation
Non-metal oxide + water → nonmetal oxyacid

Metal oxide + water → the metal hydroxide

4) Metal carbonate formation
Metal oxide + carbon dioxide → metal carbonate

Decomposition Reactions
XY → X + Y ( + Z )
1) Binary Compounds decompose into two elements

2) Metal carbonates → metal oxide + carbon

3) Metal hydrogen carbonates (bicarbonates) → metal carbonate + water+ carbon dioxide

4) Metal nitrate → into metal nitrite and oxygen gas

5) Metal hydroxide → into metal oxide and water

Metal chlorates → metal chloride + oxygen gas

Double Displacement Precipitation Reactions
- Must have percipitates
- Need to use solubility table to predict if these reactions will or occur.
WX(aq) +YZ(aq) → WZ + YX
1) Double Displacement Neutralizaton Reaction
acid + base → salt +water

2) Neutralization – Decomposition Reactions (also called Double Displacement neutralization reactions producing gas)
- Acids mixed with carbonates or bicarbonates will undergo a double displacement reaction producing a salt and carbonic acid (H2CO3).
- The carbonic acid decomposes
into water and carbon dioxide gas. The carbonic acid
intermediate is not usually written in the chemical
equation.

- Other reactions of this type include a sulphite or a hydrogen sulphite mixed with acid.
Singel Displacement Reaction
X + YZ → XZ + Y
- Some metals will react with water. These are indicated on the metal activity series. If a metal reacts with water the products are the metal hydroxide and hydrogen gas.
Metal + water → metal hydroxide + hydrogen gas

Combustion of Organic Compounds Containing only C and H or only C, H and O
If oxygen supply is limited, products are carbon monoxide and water; carbon and water; or carbon dioixde, carbon monoxide, carbon, and water

Unit 1|Thermochemistry
course pack
thermo data table
Energy Definitions
low temperture = low movement
high temperture = high movement
Specific Heat Specific heat is a physical property of matter. It is defined as the energy needed to raise the temperature of one gram of substance by one ℃
$Specific Heat =\frac{energy\ absorbed}{mass\ of\ sample\times temperture\ change\ in\ _{}^{o}\textrm{C}}$ or $c=\frac{q}{m\times \Delta T}$
Rearranging the equation $q=m\times c\times\Delta T$
Molar heat capacity: is the energy required to raise the temperature of on mole of a substance by 1 ℃(J/mol×℃)
Calorimetry
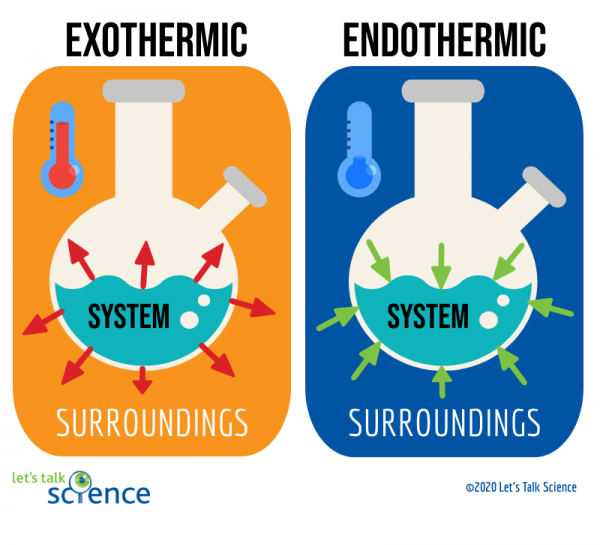
- Exothermic qsys <0
- Endothermic qsys >0
- qsys = -qsurr
Enthalpy Changes
- q for the system is positive if energy goes into the system and the temperature of the surroundings decreases.
- q for the system is negative if the system loses heat to the surrounding and the temperature of the surroundings increases
- the overall energy must be conserved qsys = -qsurr
When a physical or chemical change occurs that releases or absorbs energy
- thermal energy from the surroundings is converted to bond energy for the system in endothermic reactions
- bond energy from the system is converted to thermal energy in the surroundings in an exothermic reactions
- The overall energy must be conseved ΔHsystem = -qsurroundings
- ΔH is the transfer of energy in a reaction
- The units of enthalpy are kilojoules (kJ)
- If the amount of substance that reacted is given, the enthalpy can be expresses as a molar quantity of molar enthalpy (ΔHrxn).
- n × ΔHrxn = ΔHsystem
- The units of molar enthalpy are kilojoules per mol (kJ/mol)
Writing Thermochemical Equations
A thermochemical equation is a clear way of communicationg informations about the enthalpy change in a reaction
Ex. The synthesis of water from its elements is an exothermic reaction and can be represented by two thermochemical equations.
Hess’s law
In going from a particular set of reactants to
a particular set of products, the change in enthalpy is the same whether the reaction takes place in one
step or in a series of steps.
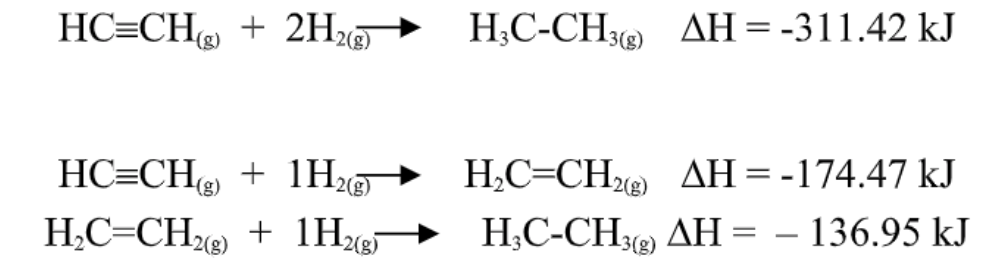
The first equation equals to the sum of the latter 2 equations.
Standard Enthalpies of Formation
Formation reactions are reactions in which a compound is formed from its element.
Ex.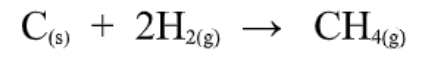
The superscript °, refers to a specific set of conditions. Standard ambient temperature (in SATP) is 25 °C = 298 K.
For a compound:
- for a gaseous substance is at a pressure of 1 atm or 101.3 kPa.
- for non-gaseous pure substances, the standard state is pure liquid or solid
- for a dissolved substance in solution, the standard state is at a concentration of exactly 1.0 M.
For an element:
- the standard state of an element is the form in which the element exists under STAP conditions of 1 atm (101.3 kPa) and 25°C = 298K.
Standard Enthalpy Change, ΔH°, is the enthalpy change that accompanies a reaction when the
reactants and products are in their standard states.
Standard Enthalpy of Formation, ΔH°f , is the enthalpy change that results when one mole of the substance is formed from its elements with all substances in their standard states.
The standard enthalpies of formation of for any element already in its standard states is zero.
Elements → 1Compound
Using Standard Heats of Formation in Hess’s Law
- reactants broken down → into their elements in standart state
- elements in standard state assembled → into the products in their standard states
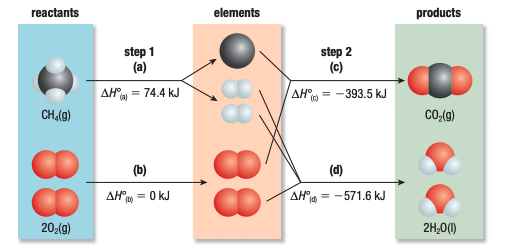
ΔH° = ∑nΔH°fproducts - ∑nΔH°freactants
Calculating ΔH using Bond Energies
Bond energy is the average energy needed to break a given type of bond.
ΔH° = ∑n × D(bonds broken) - ∑n × D(bonds formed)
D = bond energy per mole of bonds
n = moles (based on the equation coefficients
Thermodynamics, Entropy and Gibb’s Free Energy
Entropy - Molecular randomness or disorder
The symbol of entropy if ΔS (Unit: J/mol·K
- ΔS is (+) when randonmness or disorder of a system increases
- ΔS is (-) when randonmness or disorder of a system decreases
ΔS° = ∑n×ΔS°f(products) - ∑n×ΔS°f(reactants)
The value ΔG is a measure of the maximum amount of work a system can do as it approaches the end of reaction.
ΔG°=ΔH°-TΔS°
ΔGrxn° = ∑n×ΔG°f(products) - ∑n×ΔG°f(reactants)
ΔG° is negative (-) for spontaneous reaction
ΔG° is positive (+) for non-spontaneous reaction
ΔG° = 0 for a system that has both the forward and reverse reaction occuring at the same rate so the amount of products and reactants do not change
Reaction Kinetics-Factors Affecting Rate
- Nature of the reactant
- Concentration
- Surface area
- Temperature
- Catalysts
Theories of Reaction Rates
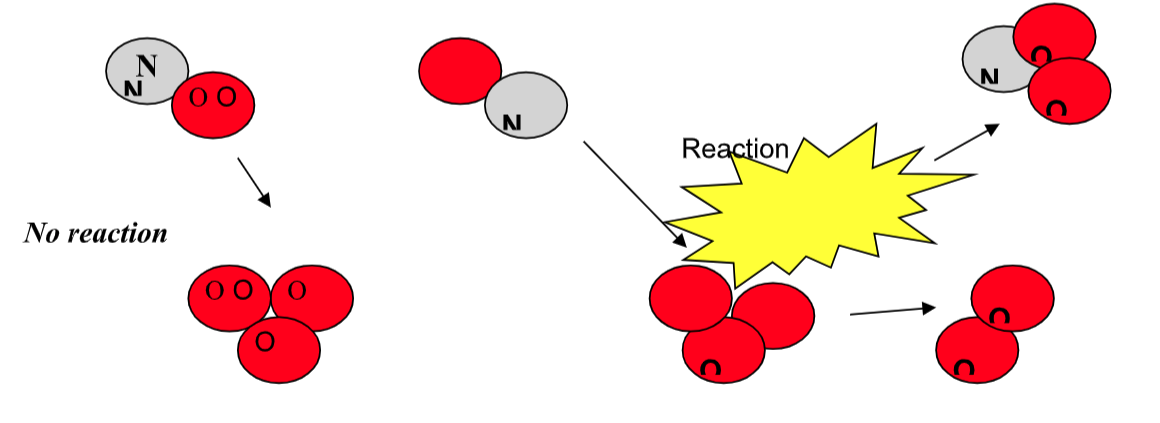
Factors Influencing Collision
1) Orientation
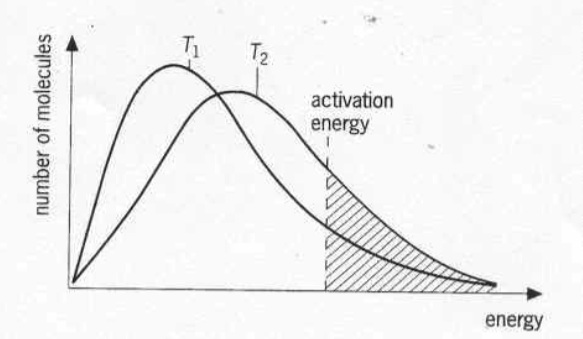
2) Energy of colliding molecules
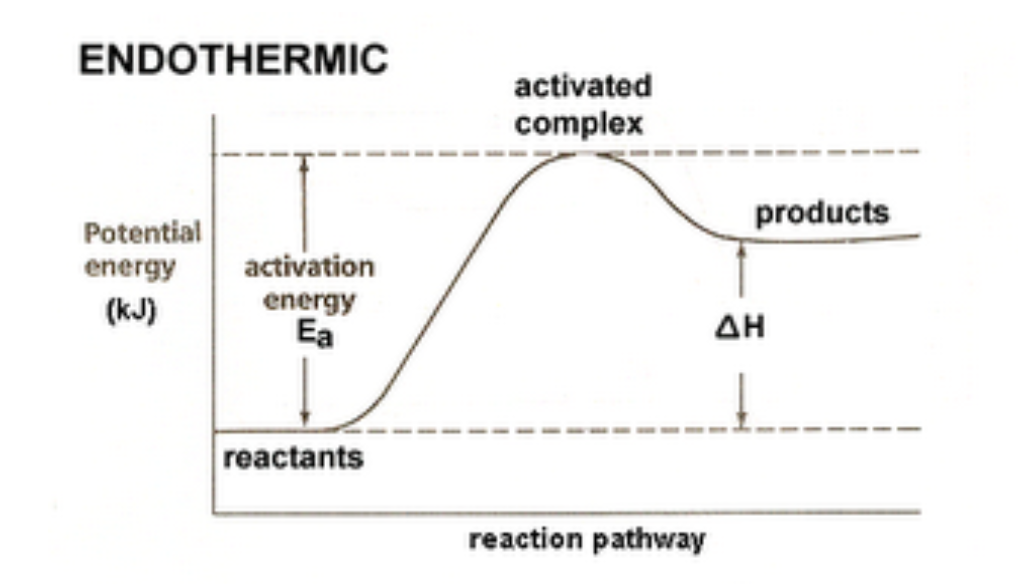
Activation Energies and Transition States
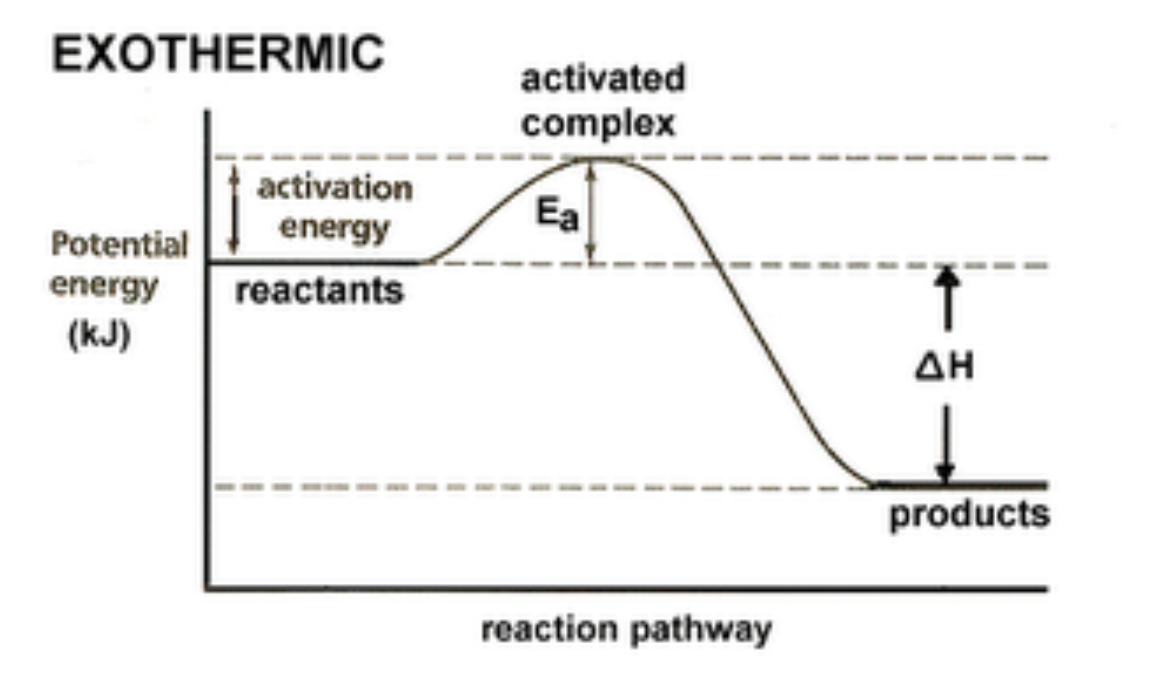
An effective collision is one in which the
reactants collide with the correct orientation and energy to form an activated complex that leads to the
formation of products.
1) Energy Diagram for an Exothermic Reaction
2) Energy Diagram for an Endothermic Reaction
Catalysts
A catalyst is a substance that increases the reaction rate without increasing the number of collisions and without being consumed itself.
- 1st Kind
$A+B\overset{y}{\rightarrow}C+D$
y is a catalyst (usually heterogeneous)
- 2nd Kind
$A+y\rightarrow Ay$ (Ay is an intermediate)
$Ay+B\rightarrow C+D+y$
y is a catalyst (usually homogeneous)
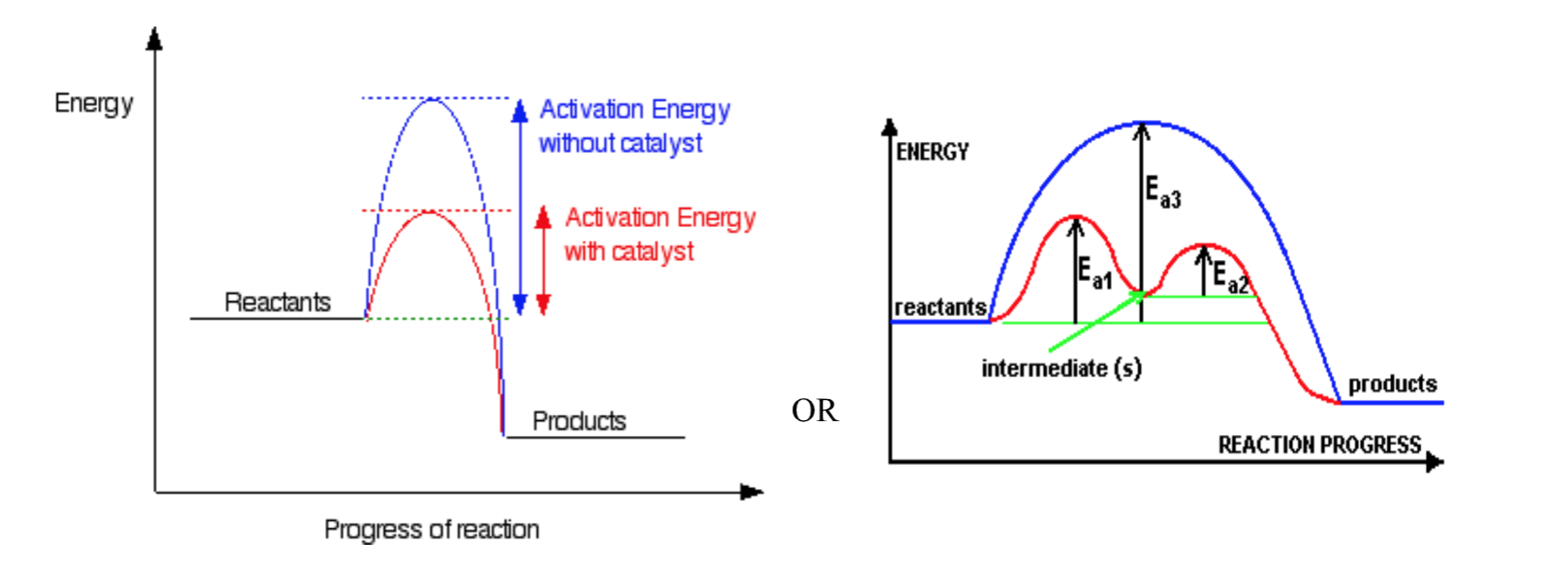
Reaction Mechanisms
Reaction Mechanisms:
Reactions can be classified as being either simple or complex. A complex reaction consists of a number of steps. Each of these steps involves one collision and is known as an elementary step. A simple reaction consists of one elementary step. The step or series of steps that make up a reaction is known as the mechanism of that reaction. From a reaction equation it is not possible to determine if it is simple or complex.
Rate Determining Step:
Every mechanism has a rate-determining step. This is the slowest step in the reaction. If the first elementary step of the mechanism is the rate-determining step, then we can say that the overall reaction rate depends on this initial step and on the concentrations of the species involved in this step. Any subsequent step is faster and does not affect the overall rate.
| Elementary step | Elementary step |
|---|---|
| A → products | Rate = k[A] |
| A + A → products | Rate = k[A]2 |
| A + B → products | Rate = k[A][B] |
Unit 2|Atomic Structure
course pack|Part 1
course pack|Part 2
Orbital Shapes
The shapes of the orbitals are determined by the angular momentum
quantum number l and the corresponding letter designations s, p, d and f.

Electron Configurations

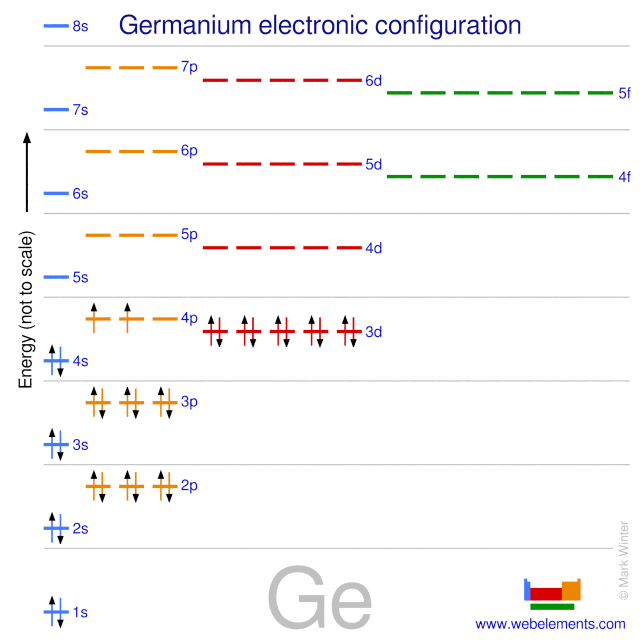
Ex: 32Ge
1s22s22p63s23p64s23d104p2(filling order) is arranged to
1s22s22p63s23p63d104s24p2(shell order)
Methods of Expressing Electron Configuration
1) filling order
Ex: 32Ge
1s22s22p63s23p64s23d104p2
2) shorthand electron configuration
Ex: 32Ge
[K] 4s23d104p2
3) orbital diagram
Ex: 32Ge
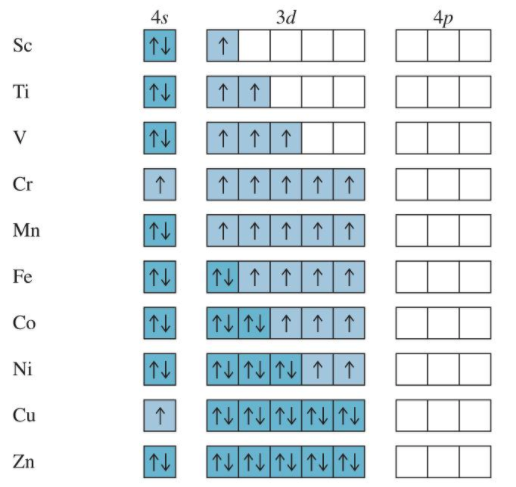
Orbital Filling Irregularities
Since he energy levels of some orbitals are so close there are irregularities in the order of electron filling. For example with the transition metals Cr and Cu the decrease in energy from having an orbital with unpaired spins causes an unusually order of filling.